Class 6 Science Chapter - 3 Separation of Substances Notes, Previous Year Questions & NCERT Solutions
Class 6 Science
Chapter - 3
Separation of Substances
Notes, Previous Year Questions & NCERT Solutions
--Notes--
A substance can be classified into: Mixture and a pure substance.
Mixtures:- A mixture is a material made up of two or more different substances which are mixed but are not combined chemically.
Separation:- Separation is the process of separating one or more components from a mixture. For example, distillation, sedimentation, filtration etc.
On a day-to-day basis, we are faced with various instances when we are required to separate substances from one another. It is usually because of one or all three of the following reasons:-
To separate two dissimilar but useful elements like in the case of butter and milk. Milk is churned in order to obtain butter.
To segregate useless elements from the useful ones like in the case of separating tea leaves from tea.
To remove and discard impurities or potentially harmful substances like picking out small pieces of stones and other impurities from rice and wheat.

Figure 1 Separating tea leaves from tea
Methods of Separation

Figure 2 Methods of Separation
Handpicking: The simple process of separating slightly bigger sized harmful substances or impurities like small pieces of stones, husk and dirt from grains of wheat, pulses and rice is called handpicking. In situations when the quantity of such impurities is not very large, handpicking turns out to be a time-saving and convenient procedure of separating substances.

Figure 3 A group of individuals separating two types of grains
Threshing: After the crop is harvested, stalks are left to dry under the sun. A single stalk has some 100 pieces of grain seeds joined to it. It is manually impossible to pluck each grain seed which is very small in size from the stalk and hence handpicking as a method of separation does not work here. That is why we use a method called threshing to separate these grain seeds.
Threshing can be defined as the process of separating the edible part i.e. grain seeds from the stalk by either with the help of machines, bullocks or sometimes by beating them.
Figure 4 a) Threshing by hand b) Threshing by machine
Winnowing: Even when threshing is done, husk or chaff is still attached to the grain seed and since the size of the two is quite similar, handpicking does not work and neither does threshing. Hence, a method called winnowing can be used.
Winnowing can be defined as the method of separating lighter husk particles and heavier grain seed components by blowing a current of air through them. The lighter husk particles are carried away by the wind and the grain seeds get separated. This husk can be further used as fodder for the cattle.

Figure 5 Process of winnowing
Sieving: Sometimes even after the grain seeds have passed through the stages of threshing and winnowing, husk may still be attached to the grain or it may have collected stones and dirt in the earlier stages which need to be removed and this separation is usually done with the help of a sieve.
Sieving is a very simple, convenient and time-saving process through which particles of varying sizes can be separated from each other with the help of a sieve. A sieve is nothing but a simple device with small pores in it which allow finer materials like flour to pass through leaving behind any impurities it might contain.

Figure 6 Sieving
Sedimentation, Decantation and Filtration
Sedimentation: Sedimentation can be defined as the process through which dirt and other heavier particles in a mixture settle at the bottom of the vessel when water is added to it. When the dust and dirt particles have settled, the clear water which forms the upper layer is moved to a different container and the dirt and dust is done away with. This technique can also be used to separate two liquids which do not mix with each other (also called immiscible liquids).
Decantation: Decantation can be defined as a technique through which immiscible liquids or a liquid and a solid substance are separated. Sometimes smaller dirt particles get carried along with the water in the process of decantation which needs to be further removed. This can be achieved through the process of filtration.
Filtration: Filtration is the process through which smaller particles like dirt etc. are separated from a solution by making the solution pass through a medium (often a filter paper). This medium is such that only liquids are able to pass through it because of the presence of very tiny pores in it. The filter paper is molded to form a cone and this cone-like structure is then affixed to a funnel through which the dirty solution is allowed to pass. Sometimes, filtration can also be applied to separate pulp and seeds from the juice. It can also be used to separate cottage cheese or paneer from milk.

Figure 7 Sedimentation, Decantation and Filtration
Evaporation: Evaporation is the process of converting liquid into gas or vapour by increasing the temperature or pressure of the liquid. This process is often used to separate salt from salt water or salty sea water. Sea water has a number of salts present in it. Shallow pits called evaporation ponds are constructed and salt water is allowed to stand in these. After some time, the water gets evaporated, leaving behind the salts. Common salt is separated from this mixture upon further purification.

Figure 8 Salt Evaporation Ponds
Condensation:- Condensation is the defined as the simple process of converting gas or vapour to its liquid form by decreasing the temperature or pressure exerted on it. This is what we did when we allowed the steam to come in contact with the cold metal plate.

Figure 9 Use of more than one method of separation
Can Water Dissolve Any Amount of a Substance?
Even though water can dissolve a number of substances and solutions in it, it has a limit to how much it can dissolve. After a certain point, it stops dissolving any more of that substance and the substance collects at the bottom of the vessel. We say that the solution has become saturated.
A saturated solution is one that contains the maximum possible concentration of a particular solute i.e. it is now incapable of dissolving any more of the given solute which is in this case, salt.
A salute is defined as a very small element in a solution that is dissolved in a solution.
One way of ensuring that the given amount of water takes more salt even after it has reached its saturation point is by heating the said water. This is because heating the solution helps to increase the solubility of salt or any solute and hence more amount of the same solute can now be dissolved in the same amount of water. The solution thus prepared is called a super saturated solution.
Some Important Definitions
Churning: The process of shaking milk or cream in order to allow lighter particles to come to the surface in order to make butter is called churning.
Pure Substance: This can be defined as a substance composed of only a single type of particle.
Impure Substance: A substance composed of more than one type of particles.
Sublimation: When a solid directly gets converted into vapour, this process is known as sublimation.
Magnetic Separation: This is another method of separation which allows metals (and other articles which are attracted to a magnet) to be separated from a mixture with the help of a magnetic or by applying a magnetic force to it. For example, a mixture of salt and iron filings can be separated with the help of a magnet.
--- NCERT Solution ---
Question 1.- Why do we need to separate different components of a mixture? Give two examples.
Answer:- Among different components of mixture there are many substances which are harmful or not useful for us. To remove these harmful components we need to separate them. For example:
a) Tea leaves are separated from the liquid with a strainer while preparing tea.
b) Stone pieces from wheat, rice or pulses are picked out by hand.
Question 2.- What is winnowing? Where is it used?
Answer:- Winnowing is used to separate heavier and lighter components of a mixture by wind or by blowing air. This process is used by farmers to separate lighter husk particles from heavier seeds of grain.
Question 3.- How will you separate husk or dirt particles from a given sample of pulses before cooking?
Answer:- Husk or dirt particles can be separated by winnowing, being lighter they wall fly away from pulses.
Question 4.- What is Sieving? Where can it be used?
Answer:- Sieving is a process by which fine particles are separated from bigger particles by using a sieve. It is used in flour mill or at construction sites. In flour mill, impurities like husks and stones are removed from wheat. Pebbles and stones are removed from sand by sieving.
Question 5.- How will you separate sand and water from their mixture?
Answer:- We will separate sand and water by sedimentation and decantation method. First we leave this mixture for some time. After some time, the sand which is; heavier is settled down at the bottom. After that we wall pour water into another container and the mixture will be separated.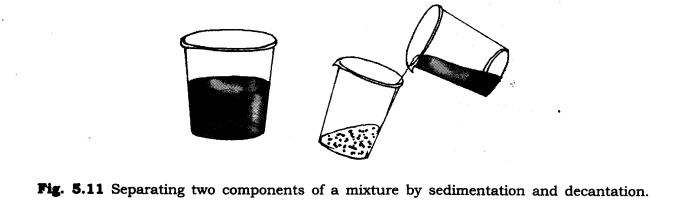
Question 6.- Is it possible to separate sugar mixed with wheat flour? If yes, how will you do it?
Answer:- Sugar can be separated from wheat flour by sieving. Due to difference in the size of particles, sugar will stay on sieve and wheat flour will pass through it.
Question 7.- How would you obtain clear water from a sample of muddy water?
Answer:- We will obtain clear water from a sample of muddy water by the process of filtration.
A filter paper is one such filter that has very fine pores in it. Figure 5.12(a, b) shows the steps involved in using a filter paper. A filter paper folded in the form of a cone is fixed in a funnel. The mixture is then poured on the filter paper. Solid particles in the mixture do not pass through it and remain on the filter.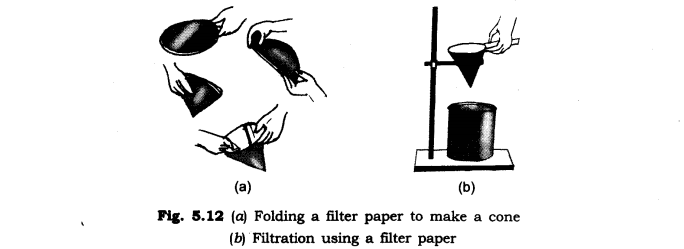
Question 8.- Fill in the blanks:
a) The method of separating seeds of paddy from its stalks is called __________.
b) When milk, cooled after boiling, is poured onto a piece of cloth the cream (malai) is left behind on it. This process of separating cream from milk is an example of ______.
c) Salt is obtained from sea water by the process of ____________ .
d) Impurities settled at the bottom when muddy water was kept overnight in a bucket. The clear water was then poured off from the top. The process of separation used in this example is called ____________.
Answer:-
a) threshing
b) filtration
b) evaporation
d) sedimentation and decantation
Question 9.- True or false?
a) A mixture of milk and water can be separated by filtration.
b) A mixture of powdered salt and sugar can be separated by the process of winnowing.
c) Separation of sugar from tea can be done with filtration.
d) Grain and husk can be separated with the process of decantation.
Answer:- a) False b) False c) False d) False
Question 10.- Lemonade is prepared by mixing lemon juice and sugar in water. You wish to add ice to cool it. Should you add ice to the lemonade before or after dissolving sugar ? In which case would it be possible to dissolve more sugar ?
Answer:- We should add ice after dissolving sugar. When the temperature is high then more sugar can be dissolved. After mixing ice it gets cool and less sugar will dissolve in it.
--- Previous Year Questions ---
Question 1.- What is threshing?
Answer:- Threshing is a process that is used to separate grain from stalks. In this process the stalks are beaten to free the grain seeds. Sometimes threshing is done with the help of bullocks. Machines are also used to thresh large quantities of grain.
Question 2.- Describe the method to obtain salt from sea water.
Answer:- Sea water contains many salts mixed in it. One of them is common salt, when sea water is allowed to stand in shallow pits, water gets evaporated by sunlight and slowly turns into water vapour. In a few days, the water evaporates completely leaving behind the solid salts. Common salt is then obtained from this mixture of salts by further purification.
Question 3.- What is decantation?
Answer:- Decantation is a process, of separation of insoluble solids from liquid. The suspension of solid particles in liquid is allowed to stand for some time. The solid particles then settle down at the bottom of the container and clean water goes up. Without disturbing the settled particles the clean water is transferred into other container.
Question 4.- Where is decantation used? Give two examples.
Answer:-
i) Decantation is used to separate insoluble solids or liquid from liquid. Rain water is a mixture of mud and water. It is purified by decantation.
ii) Oil and water also get separated by this method because oil floats up.
Question 5.- How will you prepare cheese (paneer)?
Answer:- For making paneer, a few drops of lemon juice sire added to milk as it boils. This gives a mixture of particles of solid paneer and liquid. The paneer is then separated by filtering the mixture through a fine cloth or strainer.
Question 6.- Explain the method that can be used for separating the following mixture:
i) Sand and husk
ii) Wheat, sugar and stalk
iii) Water and petrol
iv) Rice and salt
v) Sand and salt
Answer:-
i) Mixture of sand and husk:- Sand and husk can be separated by the method of winnowing.
ii) Mixture of wheat, sugar and stalk:- For separating stalk from the mixture we should follow the winnowing method because milk is lighter than other two components and get separated. Wheat and sugar can be separated by sieving because they are in different sizes.
iii) Mixture of water and petrol:- Water does not dissolve in petrol. So, it can be separated by the use of separating funnel.
iv) Mixture of rice and salt:- Rice and salt can be separated by sieving.
v) Mixture of sand and salt:- Sand and salt is mixed with water, salt dissolves in water and sand can be separated solution by sedimentation and decantation followed by filtration. After that using evaporation common salt is separated.
Give Us Your Feedback/Suggestions
- By Durgesh Pandey
(Eklavya Coaching Institute)
📞 8376976688, 9310533915
H-2/25, Gali No-23, Kunwar Singh Nagar, Nangloi, New Delhi -110041

.jpg)




Comments
Post a Comment