Class - 9 Science (Chemistry) Chapter - 4 Structure of Atom Notes, NCERT Solutions & Frequently Asked Questions
Class - 9
Science (Chemistry)
Chapter - 4
Structure of Atom
Notes, NCERT Solutions & Frequently Asked Questions
-- Notes --
Atoms are the basic building blocks of matter.
Different kinds of matter exist because there are different kinds of atoms present in them.
Charged Particles in Matter
Whenever we rub two objects together, they become electrically charged. This is because atoms contain charged particles in them. Therefore, atoms can be divided further into particles i.e proton, electron and neutron.
Protons were discovered by Ernest Rutherford, in his famous gold foil experiment.
Electrons were discovered by J.J. Thomson, in his cathode ray tube experiment.
Neutrons were discovered by James Chadwick.

Atoms consist of protons and electrons in a balanced proportion.
Protons exist in the interiors of the atom and electrons exist in the exteriors of the atom. Therefore, electrons can be removed from an atom.
Failure of Dalton’s Atomic Theory
The postulates of the atomic theory by John Dalton
The matter is made up of tiny particles called Atoms that cannot be divided.
Atoms are never formed or destroyed during a chemical reaction.
Atoms of an element exhibit same nature. They have the same size, mass, and character.
Atoms of different elements exhibit variant nature. They do not have same characteristics.
Atoms form compounds by combining in a ratio of whole numbers.
A compound contains a constant number and kinds of atoms
Dalton suggested that atoms can neither be created nor destroyed and are indivisible. But the discovery of electrons and protons in atoms lead to failure of this aspect of Dalton’s theory.
Thomson’s Model of an Atom
According to J.J. Thomson, the structure of an atom can be compared to Christmas pudding where electrons are present inside a positive sphere.
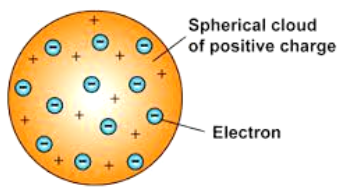
An atom is composed of a positively charged sphere in which electrons are embedded.
Atom is neutral as the positive and negative charged are equal in proportion.
Rutherford’s Model of an Atom
Rutherford’s Experiment
He experimented with thin gold foil by passing alpha rays through it.
He expected that the gold atoms will deflect the Alpha particles.

| Observations | Inferences |
Alpha particles which had high speed moved straight through the gold foil | Atom contains a lot of empty space |
Some particles got diverted a by slide angles | Positive charges in the atom are not occupying much of its space |
Only one out of 12000 particles bounced back | The positive charges are concentrated over a particular area of the atom. |
Thus, Rutherford gave the nuclear model of an atom based on his experiment which suggests that -
Atoms contain a lot of unoccupied space
There is a heavily positively charged substance present in the center of the atom which is called the nucleus
The nucleus contains an equal amount of positive and negative charge.
The Nucleus of an Atom
The nucleus id located at the center of the atom.
All the mass of the atom is because of the nucleus.
The electrons revolve around the nucleus in circular parts which are called Orbits
If we compare the size of the atom and nucleus, the nucleus is much smaller than the atom.

Drawbacks of the Nuclear Model of an Atom
The Nuclear Model of the Atom failed to explain how an atom remains stable despite having positive and negative charges present in it. Maxwell has suggested a theory according to which if any charged particle moves in a circular motion it radiates energy. So, if electrons start moving in a circular motion around the nucleus they would also radiate some energy which would decrease at the speed of the electrons. As a result, they would fall into the nucleus because of its high positive charge.
What are nucleons? – Protons and Neutrons are collectively called as Nucleons.
Bohr's Model of an Atom
Bohr suggested that –
Electrons spin around the nucleus in an individualized separate path or unattached orbit.
The electrons do not emit any energy while moving Indies special orbits.
These orbits are also called as Energy Levels.
They are represented using letters or numbers as shown in the figure below –

The Neutrons
J. Chadwick discovered that there is another sub-atomic particle present in the atom. This particle carries no charge and is known as a Neutron. Therefore, we can conclude that atom consists of three types of particles -
| Electrons | which carry a negative charge |
| Protons | which carry a positive charge |
| Neutrons | they are neutral |
The distribution of electrons in different shells or orbits
If Orbit number = n
Then number of electrons present in an Orbit = 2n2
So, for n =1
Maximum electrons present in shell – K = 2 * (1)2 = 2
The outermost shell can contain at most 8 electrons.
The shells in an atom are filled in sequence.
Thus, until the inner shells of an atom are filled completely the outer shells cannot contain any electrons.
Valency
Valence Electrons – Electrons existing in the outermost orbit of an atom are called Valence Electrons.
The atoms which have completely filled the outermost shell are not very active chemically.
The valency of an atom or the combining capacity of an atom is given by the number of elements present in the outermost shell.
For Example, Helium contains two electrons in its outermost shell which means its valency is two. In other words, it can share two electrons to form a chemical bond with another element.
What happens when the outermost shell contains a number of electrons that are close to its maximum capacity?
Valency in such cases is generated by subtracting the number of electrons present in the outermost orbit from octet (8). For example, oxygen contains 6 electrons in its outermost shell. Its valency is calculated as: 8 – 6 = 2. This means oxygen needs two electrons to form a bond with another element.
Atomic Number of an Element
Atomic Number (Z) = Number of protons in an atom
Mass Number of an Element
Mass Number = Number of protons + Number of neutrons

Isotopes
The atoms of an element can exist in several forms having similar atomic numbers but varying mass numbers.
Isotopes are pure substances.
Isotopes have a similar chemical nature.
Isotopes have distinct physical characteristics.

Where can we use Isotopes?
1. The fuel of Nuclear Reactor – Isotope of Uranium
2. Treatment of Cancer – Isotope of Cobalt
3. Treatment of Goiter – Isotope of Iodine
Example: Consider two atomic species namely U and V. Are they isotopes?
| U | V | |
| Protons | 5 | 5 |
| Neutrons | 5 | 6 |
| Mass Number | 5 + 5 = 10 | 5 + 6 = 11 |
| Atomic Number | 5 | 5 |
From the above example, we can infer that U and V are isotopes because their atomic number is the same.
Isobars
The atoms of several elements can have a similar mass number but distinct atomic masses. Such elements are called Isobars.

***** NCERT Solution *****
Exercise-4.1 Page: 47
Question 1.- What are the canal rays?
Solution:- The radiations that are positively charged are canal rays. This discovery was crucial in the discovery of another subatomic particle that was positively charged – the proton.
Question 2.- If an atom contains one electron and one proton, will it carry any charge or not?
Solution:- Since a proton is a positively charged particle and an electron is a negatively charged particle, the net charge becomes neutral as both particles neutralize each other.
Exercise-4.2 Page: 49
Question 1.- On the basis of Thompson’s model of an atom, explain how the atom is neutral as a whole.
Solution:- As per Thompson’s model of an atom :-
i) An atom contains a positively charged sphere in which the negatively charged electrons are implanted.
ii) Electrons and protons are equal in magnitude; hence, an atom, on the whole, is electrically neutral.
Question 2.- On the basis of Rutherford’s model of an atom, which subatomic particle is present in the nucleus of an atom?
Solution:- As per Rutherford’s model of an atom, the positively charged protons are the ones that are present in the atom.
Question 3.- Draw a sketch of Bohr’s model of an atom with three shells.
Solution:-

Question 4.- What do you think would be the observation if the ∝– particle scattering experiment is carried out using a foil of a metal other than gold?
Solution:- In the ∝ – particle scattering experiment, when any other metal foil is used instead of gold, the observation would remain the same. This is because the structure of an atom, when considered individually, remains the same.
Exercise-4.2.4 Page: 49
Question 1.- Name the three subatomic particles of an atom.
Solution:- An atom consists of three subatomic particles:
- Protons – Positively charged
- Electrons – Negatively charged
- Neutrons – Neutral in nature (no charge)
Question 2.- Helium atom has an atomic mass of 4 u and two protons in its nucleus. How many neutrons does it have?
Solution:- Given: Atomic mass of helium atom = 4u, 2 protons in helium nucleus
Atomic mass = number of protons + number of neutrons
4 = 2 + number of neutrons
Number of neutrons = 4 – 2 = 2
Hence, Helium has 2 neutrons.
Exercise-4.3 Page: 50
Question 1.- Write the distribution of electrons in Carbon and Sodium atoms.
Solution:- A carbon atom contains a total of 6 electrons. The following equation describes the electron distribution in a carbon atom: first orbit or K-shell = 2 electrons; second orbit or K-shell = 2 electrons; third orbit or K-shell = 2 electrons; fourth orbit or K-shell
L-shell or second orbit = 4 electrons
We can also express the electron distribution in a carbon atom as 2, 4.
In a sodium atom, there are 11 total electrons. The electron distribution in the sodium atom is described by: first orbit or K-shell = 2 electrons; second orbit or K-shell = 2 electrons; third orbit or K-shell = 2 electrons; fourth orbit or K-shell = 2
L-shell or second orbit = 8 electrons
M-shell or third orbit = 1 electron
Alternatively, we can express the electron distribution in a sodium atom as 2, 8, 1.
Question 2.- If the K and L shells of an atom are full, then what would be the total number of electrons in the atom?
Solution:-
K shell can hold 2 electrons.
L shell can hold 8 electrons.
Hence, when both the shells are full, the total number of electrons present in the atom = 2+8 = 10 electrons.
Exercise-4.4 Page: 52
Question 1.- How will you find the valency of chlorine, sulphur and magnesium?
Solution:- We know that an element’s valency refers to its proclivity for accepting or losing electrons in order to complete its octet and achieve a stable electronic state.
It is the smallest number of electrons that must be added or removed to entirely occupy an element’s outermost shell.
Mathematically, if an atom’s outermost shell contains 4 or fewer electrons, the element’s valency is equal to the number of electrons present in the outermost shell; if it contains more than 4, the valency is determined by subtracting the total number of electrons present in the outermost shell from 8.
Calculation of valency:
Valency of chlorine:
The electronic configuration of chlorine = 2, 8, 7
Chlorine has 7 (more than 4) electrons in its outermost shell.
Therefore, the valency of chlorine = 8 – the number of electrons in the outermost shell
= 8−7
= 1
Valency of Sulphur:
The electronic configuration of Sulphur = 2, 8,6
Sulphur has 6 (more than 4) electrons in its outermost shell.
Therefore, the valency of chlorine = 8 – the number of electrons in the outermost shell
= 8−6
= 2
Valency of magnesium:
The electronic configuration of Magnesium = 2, 8, 2
Magnesium has 2 (less than 4) electrons in its outermost shell.
Therefore, the valency of magnesium= Number of electrons in its outermost shell
= 2
Exercise-4.5 Page: 52
Question 1.- If the number of electrons in an atom is 8 and the number of protons is also 8, then
i) What is the atomic number of the atom? and
ii) What is the charge on the atom?
Solution:-
Given: Number of electrons = 8
Number of protons = 8
i) The atomic number of an atom is the same as the number of protons in that atom; hence, its atomic number is 8.
ii) In an atom, the number of protons is equal to the number of electrons. Hence, both the charges – positive and negative – neutralize each other. Therefore, the atom does not possess any charge.
Question 2.- With the help of the given table, find out the mass number of oxygen and sulphur atom.
Solution:-
a) To find the mass number of Oxygen,
Number of protons = 8
Number of neutrons = 8
Atomic number = 8
Atomic mass number = Number of protons + number of neutrons = 8 + 8 = 16
Therefore, the mass number of oxygen = 16
b) To find the mass number of Sulphur,
Number of protons = 16
Number of neutrons = 16
Atomic number = 16
Atomic mass number = Number of protons + number of neutrons = 16 + 16 = 32
Exercise-4.6 Page: 53
Question 1.- For the symbols H, D and T, tabulate three subatomic particles found in each of them.
Solution:- The following table depicts the subatomic particles in Hydrogen (H), Deuterium (D), and Tritium(T).
Question 2.- Write the electronic configuration of any one pair of isotopes and isobar.
Solution:-
a) Isotopes:- Isotopes are atoms which have the same number of protons, but the number of neutrons differs. This leads to the variation in mass number too.
Example: Carbon molecule exists as 6C12 and 6C14, but when their electronic configuration is noticed, both have K-2; L-4
b) Isobars:- Isobars are atoms which have the same mass number but differ in atomic number. The electronic configuration of an isobar pair is as follows:
Example: Electronic configuration of 20Ca40 – K-2; L-8; M-8; N- 2
Electronic configuration of 18Ar40 – K-2; L-8; M-8
Exercise Page: 54
Question 1.- Compare the properties of electrons, protons and neutrons.
Solution:-
Question 2.- What are the limitations of J.J.Thomson’s model of the atom?
Solution:- The following are the limitations of J.J. Thomson’s model of an atom:
- The model failed to explain the outcome of alpha particle scattering, which was conducted by Rutherford. The model failed to depict why the majority of these alpha particles pass through the gold foil, while some diverted through small and big angles, while some others rebound completely, returning on their path.
- It did not provide any experimental evidence and was established on imagination.
Question 3.- What are the limitations of Rutherford’s model of the atom?
Solution:- The following are the limitations of Rutherford’s model of the atom:
- There is no expected stability in the revolution of the electron in a circular orbit.
- Charged particles radiate energy when accelerated, thus causing the revolving electrons to lose energy and would fall into the nucleus.
- Hence, atoms must be highly unstable. The matter would not exist in its known form, which clearly is an assumption as atoms are highly stable.
Question 4.- Describe Bohr’s model of the atom.
Solution:- 𑇐 An atom holds the nucleus at the centre.
- Negatively charged electrons revolve around the nucleus.
- The atoms in it contain distinct orbits of electrons.
- Electrons do not radiate energy when they are in their orbits.
- The distinct orbits are named K, L, M, and N orbits. Numbers used to denote them are n=1, 2, 3, 4

Question 5.- Compare all the proposed models of an atom given in this chapter.
Solution:-
Thomson’s Model of Atom.

Rutherford’s Model of Atoms.

Bohr’s model of the atom.

Question 6.- Summarise the rules for the writing of the distribution of electrons in various shells for the first eighteen elements.
Solution:-
- The maximum number of electrons that can be accommodated in a shell is given by the formula: 2n2, where n= 1, 2, 3…
- The maximum number of electrons in different shells are:
K shell – n=1 ; 2n2 = 2(1)2 = 2
L shell – n=2 ; 2n2 = 2(2)2 = 8
M shell – n=3 ; 2n2 = 2(3)2 = 18
N shell- n=4 ; 2n2 = 2(4)2 = 32
- The outermost orbit can be accommodated with 8 electrons at the maximum.
- The electrons are not taken in unless the inner shells are filled, which are filled step-wise; hence, the highest element has K-2; L-8; M-8 distribution of electrons.
Question 7.- Define valency by taking examples of silicon and oxygen.
Solution:- The definite combining capacity of the atoms of each element, wherein electrons are lost, gained or shared to make the octet of electrons present in the outermost shell, is defined as valency. To measure valency, we can figure out the number of electrons that are required to complete the shell in which it is contained or losing excess electrons, if present, once the filling is complete.
Example: To find the valency of silicon,
The atomic number of silicon is 14.
The number of electrons is equal to the number of protons in silicon, i.e., 14.
The distribution of electrons in silicon atoms is K – 2, L – 8, M – 4
Hence, from the distribution of silicon, it is clearly evident that to fill the M shell, 4 electrons are required. Therefore, its valency is 8-4=4
To find the valency of oxygen,
The atomic number of oxygen is 8.
The number of electrons is equal to the number of protons in oxygen, i.e., 8.
The distribution of electrons in oxygen atom is K – 2, L – 6
Hence, from the distribution of oxygen, it is clearly evident that to fill the M shell, 6 more electrons are required. Therefore, its valency is 8-6=2
Question 8.- Explain with examples
i) Atomic number,
ii) Mass number,
iii) Isotopes and
iv) Isobars.
Give any two uses of isotopes.
Solution:- i) The number of positively charged protons present in the nucleus of an atom is defined as the atomic number and is denoted by Z. Example: Hydrogen has one proton in its nucleus; hence, its atomic number is one.
ii) The total number of protons and neutrons present in the nucleus of an atom is known as the mass number. It is denoted by A. 20Ca40 . The mass number is 40. The atomic number is 20.
iii) The atoms which have the same number of protons but a different number of neutrons are referred to as isotopes. Hence, the mass number varies.
Example: The most simple example is the Carbon molecule which exists as 6C12 and 6C14
iv) Isobars: Isobars are atoms which have the same mass number but differ in atomic number.
Examples are, 20Ca40and 18Ar40
Uses of isotopes
- The isotope of the Iodine atom is used to treat goitre, an iodine-deficient disease.
- In the treatment of cancer, an isotope of cobalt is used.
- Fuel for nuclear reactors is derived from the isotopes of the Uranium atom.
Question 9.- Na+ has completely filled K and L shells. Explain.
Solution:- The atomic number of sodium is 11. It has 11 electrons in its orbitals, wherein the number of protons is equal to the number of electrons. Hence, its electronic configuration is K-2 ; L-8 ; M-1 ; The one electron in the M shell is lost, and it obtains a positive charge since it has one more proton than electrons and obtains a positive charge, Na+ . The new electronic configuration is K-1; L-8, which is the filled state. Hence, it is very difficult to eliminate the electron from a filled state as it is very stable.
Question 10.- If the bromine atom is available in the form of, say, two isotopes 35Br79 (49.7%) and 35Br81 (50.3%), calculate the average atomic mass of the Bromine atom.
Solution:- The atomic mass of an element is the mass of one atom of that element. Average atomic mass takes into account the isotopic abundance.
Isotope of bromine with atomic mass 79 u = 49.7%
Therefore, Contribution of 35Br79 to atomic mass = (79 × 49.7)/100
⇒ 39.26 u
Isotope of bromine with atomic mass 81 u = 50.3%
Contribution of 35Br81 to the atomic mass of bromine = (81 × 50.3)/100
⇒ 40.64u
Hence, the average atomic mass of the bromine atom = 39.26 + 40.64 u = 79.9u
Question 11.- The average atomic mass of a sample of element X is 16.2 u. What are the percentages of isotopes 8X16 and 8X18 in the sample?
Solution:- Let the percentage of 8X16 be ‘a’ and that of 8X18 be ‘100-a’.
As per the given data,
16.2u = 16 a / 100 + 18 (100-a) /100
1620 = 16a + 1800 – 18a
1620 = 1800 – 2a
a = 90%
Hence, the percentage of the isotope in the sample 8X16 is 90% and that of
8X18 = 100-a = 100- 90=10%
Question 12.- If Z=3, what would be the valency of the element? Also, name the element.
Solution:- Given: Atomic number, Z = 3
The electronic configuration of the element = K-2; L-1, hence its valency = 1
The element with atomic number 3 is Lithium.
Question 13.- Composition of the nuclei of two atomic species, X and Y, are given as under X Y
Protons = 6 6
Neutrons = 6 8
Give the mass numbers of X and Y. What is the relation between the two species?
Solution:- Mass number of X: Protons + neutrons = 6+6 = 12
Mass number of Y: Protons + neutrons = 6+8 = 14
They are the same element, and their atomic numbers are the same.
They are isotopes, as they differ in the number of neutrons and hence their mass numbers.
Question 14.- For the following statements, write T for true and F for false.
a) J.J. Thomson proposed that the nucleus of an atom contains only nucleons.
b) A neutron is formed by an electron and a proton combining together. Therefore, it is neutral.
c) The mass of an electron is about 1/2000 times that of a proton.
d) An isotope of iodine is used for making tincture iodine, which is used as a medicine.
Solution:- a) False b) False c) True d) False
Put a tick(✓) against the correct choice and cross(x) against the wrong choice in questions 15, 16 and 17.
Question 15.- Rutherford’s alpha–particle scattering experiment was responsible for the discovery of
a) Atomic nucleus b) Electron c) Proton d) Neutron
Solution:- a) Atomic nucleus
Question 16.- Isotopes of an element have
a) The same physical properties b) Different chemical properties
c) Different number of neutrons d) Different atomic numbers
Solution:- c) Different number of neutrons
Question 17.- Number of valence electrons in Cl– ion are
a) 16 b) 8 c) 17 d) 18
Solution:- b) 8
The electronic distribution of Cl is K-2, L-8, M-7. Valence electrons are 7; hence, chlorine gains one electron for the formation of Cl–. Therefore, its valency is 8.
Question 18.- Which one of the following is a correct electronic configuration of Sodium?
a) 2, 8 b) 8, 2, 1 c) 2, 1, 8 d) 2, 8, 1
Solution:- d) 2, 8, 1
Question 19.- Complete the following table.
Solution:-The following table depicts the missing data:
Atomic number(Z) = Number of protons
Mass number = Number of neutrons + atomic number
(or)
Mass number(A) = Number of neutrons + number of neutrons
Give Us Your Feedback/Suggestions
- By Durgesh Pandey
(Eklavya Coaching Institute)
📞 8376976688, 9310533915
H-2/25, Gali No-23, Kunwar Singh Nagar, Nangloi, New Delhi -110041



Comments
Post a Comment